
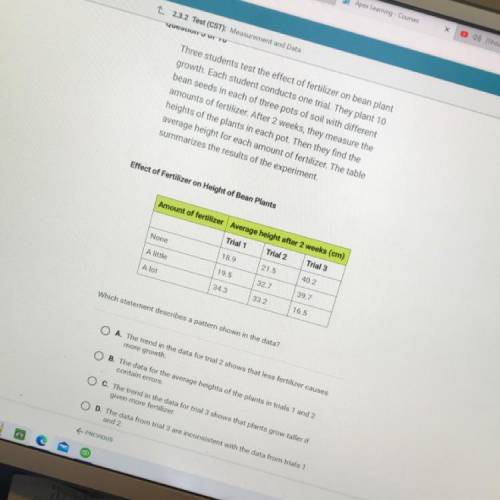
Three students test the effect of fertilizer on bean plant
growth. Each student conducts one trial. They plant 10
bean seeds in each of three pots of soil with different
amounts of fertilizer. After 2 weeks, they measure the
heights of the plants in each pot. Then they find the
average height for each amount of fertilizer. The table
summarizes the results of the experiment.
Effect of Fertilizer on Height of Bean Plants
Amount of fertilizer Average height after 2 weeks (cm)
Trial 1 Trial 2 Trial 3
None
18.9
21.5
40.2
A little
19.5
32.7
39.7
A lot
34.3
33.2
16.5
Which statement describes a pattern shown in the data?
O A. The trend in the data for trial 2 shows that less fertilizer causes
more growth
O B. The data for the average heights of the plants in trials 1 and 2
contain errors.
O C. The trend in the data for trial 3 shows that plants grow taller if
given more fertilizer.
O D. The data from trial 3 are inconsistent with the data from trials 1
and 2
lol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Three students test the effect of fertilizer on bean plant
growth. Each student conducts one trial....
Questions

Computers and Technology, 27.05.2021 15:10

Mathematics, 27.05.2021 15:10

Mathematics, 27.05.2021 15:10

Physics, 27.05.2021 15:10

Mathematics, 27.05.2021 15:10


Mathematics, 27.05.2021 15:10

English, 27.05.2021 15:10



Mathematics, 27.05.2021 15:10

Mathematics, 27.05.2021 15:10




Mathematics, 27.05.2021 15:10

Biology, 27.05.2021 15:10



Mathematics, 27.05.2021 15:10



