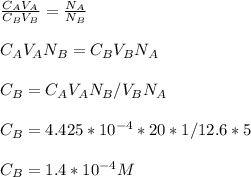Chemistry, 03.11.2020 03:20 sihamabdalla591
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O was dissolved in 200. mL of water. 20.0 mL of the solution had some acid added to it and then it reacted completely with 12.6 mL of KMnO4 solution. Calculate the concentration of the KMnO4 solution given the full REDOX equation below. 5Fe2+ + MnO4- + 8H+ --> 5Fe3+ +Mn2+ + 4H2O

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O wa...
Questions

Mathematics, 28.10.2020 22:00

Physics, 28.10.2020 22:00

Biology, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

History, 28.10.2020 22:00

Chemistry, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00



Chemistry, 28.10.2020 22:00

Health, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00


Computers and Technology, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00



History, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

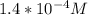
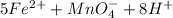 →
→ 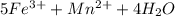
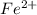 in FeSO₄(NH₄)₂SO₄*6H₂O
in FeSO₄(NH₄)₂SO₄*6H₂O 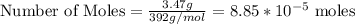
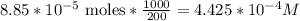
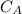 be concentration of
be concentration of 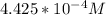
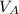 )= 20.0 ml
)= 20.0 ml
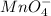 be
be 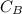 (the unknown)
(the unknown)
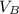 ) = 12.6 ml
) = 12.6 ml
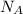 = 5 moles
= 5 moles
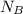 = 1 mole
= 1 mole