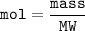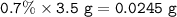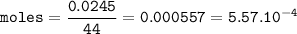Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
With white light, a team measured a 0.7% percent change with 3.5g of plant matter in a one liter con...
Questions

Mathematics, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

Advanced Placement (AP), 12.08.2020 07:01




Health, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

Health, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

Chemistry, 12.08.2020 07:01

English, 12.08.2020 07:01


Biology, 12.08.2020 07:01

History, 12.08.2020 07:01


History, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01


Biology, 12.08.2020 07:01