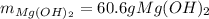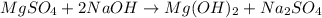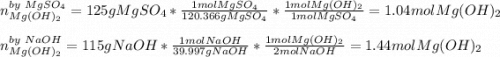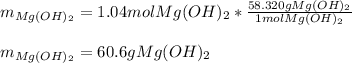Chemistry, 03.11.2020 16:40 babyleah2826
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hydroxide solution. If a solution containing 125 g of MgSO4 is added to a solution with 115 g of NaOH, what is the mass of milk of magnesia produced

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

You know the right answer?
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hyd...
Questions

Physics, 13.01.2021 23:30


Mathematics, 13.01.2021 23:30



Mathematics, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30



Social Studies, 13.01.2021 23:30



Spanish, 13.01.2021 23:30


Mathematics, 13.01.2021 23:30


Mathematics, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30