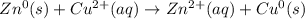Chemistry, 03.11.2020 16:50 bullockarwen
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent in the reaction of zinc with copper ions. Zn(s)+Cu2+(aq)⟶Zn2+(aq)+Cu(s) Which substance in the reaction gets reduced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent...
Questions






English, 04.12.2021 08:20

English, 04.12.2021 08:20

Mathematics, 04.12.2021 08:20

Mathematics, 04.12.2021 08:20

History, 04.12.2021 08:20


Mathematics, 04.12.2021 08:20



English, 04.12.2021 08:20



Business, 04.12.2021 08:20

Mathematics, 04.12.2021 08:20

Mathematics, 04.12.2021 08:20