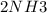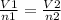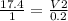Chemistry, 03.11.2020 17:00 diamoniquepowel
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L. The following reaction takes place: N2(g) + 3H2(g)2NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L...
Questions

Mathematics, 16.08.2020 01:01




History, 16.08.2020 01:01


Chemistry, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Chemistry, 16.08.2020 01:01






World Languages, 16.08.2020 01:01

History, 16.08.2020 01:01

English, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

 →
→