
Chemistry, 04.11.2020 02:30 Homepage10
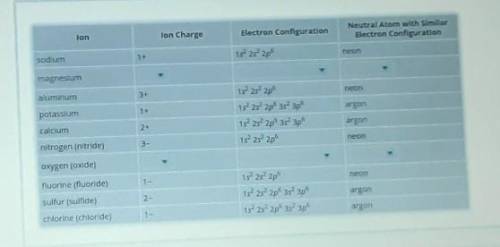
Recall that atoms gain or lose electrons to become ions. So, an ion has a different number of electrons than its corresponding neutral atom. Positive ions have lost electrons, and negative ions have gained electrons. The table includes the electron configuration of several ions. Complete the rows for magnesium and oxygen (oxide) ions by identifying the correct ion charge, electron configuration, and neutral atom with the same configuration as the ion. You may need to use online resources or the periodic table to determine the correct ion charges. Select the correct answer from each drop-down menu to complete the table.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Recall that atoms gain or lose electrons to become ions. So, an ion has a different number of electr...
Questions


Mathematics, 28.05.2020 05:58


Mathematics, 28.05.2020 05:58

History, 28.05.2020 05:58


Mathematics, 28.05.2020 05:58



Mathematics, 28.05.2020 05:58

Mathematics, 28.05.2020 05:58



Mathematics, 28.05.2020 05:58


Mathematics, 28.05.2020 05:58




Mathematics, 28.05.2020 05:58



