
Chemistry, 04.11.2020 09:10 love123jones
Solve the following problems on ideal gas equation. Practice makes perfect.
1. A toy balloon filled Analyze
with air has an
internal pressure of
1.25 atm and a
Calculate
volume of 2.50 L.
How many moles of
gas does the balloon
Answers: 1., 0.130 mol, 2.,0,012 mol, 3., 26 220 torr, 4., 1.07 atm
hold? (Assume T =
285 K)
Final Answer
2. A certain quantity of Analyze:
argon gas is under 16
torr pressure at 253K
in a 12 L vessel. How Calculate
many moles of argon
is present?
Final Answer
3. If 11.00 moles of HC1 Analyze:
gas occupies 15.00 L
at 300.0 °C, what is
its pressure in tort? Calculate
Final Answer
Analyze
4. What would be the
pressure of 6.40 g
oxygen gas in a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
Solve the following problems on ideal gas equation. Practice makes perfect.
1. A toy balloon filled...
Questions


Physics, 23.06.2019 00:30


English, 23.06.2019 00:30

Health, 23.06.2019 00:30

Mathematics, 23.06.2019 00:30




Mathematics, 23.06.2019 00:30

History, 23.06.2019 00:30

Computers and Technology, 23.06.2019 00:30

Mathematics, 23.06.2019 00:30

Physics, 23.06.2019 00:30



Computers and Technology, 23.06.2019 00:30



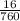 = 0.02atm
= 0.02atm 

