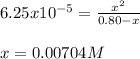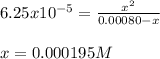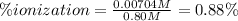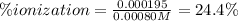Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

You know the right answer?
Be sure to answer all parts. Calculate the percent ionization of benzoic acid for the following conc...
Questions


History, 24.10.2019 02:20

Arts, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Chemistry, 24.10.2019 02:20


Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Social Studies, 24.10.2019 02:20

History, 24.10.2019 02:20

Health, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20


Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

Mathematics, 24.10.2019 02:20

English, 24.10.2019 02:20

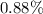
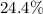
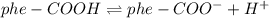
![Ka=\frac{[phe-COO^-][H^+]}{[phe-COOH]}](/tpl/images/0866/6698/da6c4.png)
 ):
):![6.25x10^{-5}=\frac{x^2}{[phe-COOH]_0-x}](/tpl/images/0866/6698/7e878.png)