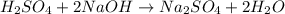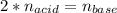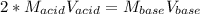Chemistry, 04.11.2020 19:00 joylsbarbour
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H2SO4). a. Write a balanced equation for the neutralizationof NaOH with H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H...
Questions

Biology, 13.10.2019 13:20


Mathematics, 13.10.2019 13:20

Biology, 13.10.2019 13:20

History, 13.10.2019 13:20

History, 13.10.2019 13:20



Computers and Technology, 13.10.2019 13:20


Arts, 13.10.2019 13:20



History, 13.10.2019 13:20

Mathematics, 13.10.2019 13:20


Mathematics, 13.10.2019 13:20



Mathematics, 13.10.2019 13:20