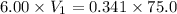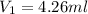Chemistry, 05.11.2020 18:30 AgarioEdit
You wish to make a 0.341 M hydroiodic acid solution from a stock solution of 6.00 M hydroiodic acid. How much concentrated acid must you add to obtain a total volume of 75.0 mL of the dilute solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
You wish to make a 0.341 M hydroiodic acid solution from a stock solution of 6.00 M hydroiodic acid....
Questions




Mathematics, 12.01.2022 07:50

SAT, 12.01.2022 07:50


Social Studies, 12.01.2022 07:50

SAT, 12.01.2022 07:50

Health, 12.01.2022 07:50



Physics, 12.01.2022 07:50



Social Studies, 12.01.2022 07:50



Geography, 12.01.2022 07:50


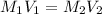
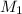 = concentration of stock
= concentration of stock  = 6.00 M
= 6.00 M
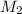 = concentration of resulting
= concentration of resulting 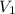 = volume of stock
= volume of stock 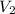 = volume of resulting
= volume of resulting 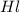 = 75.0 ml
= 75.0 ml