
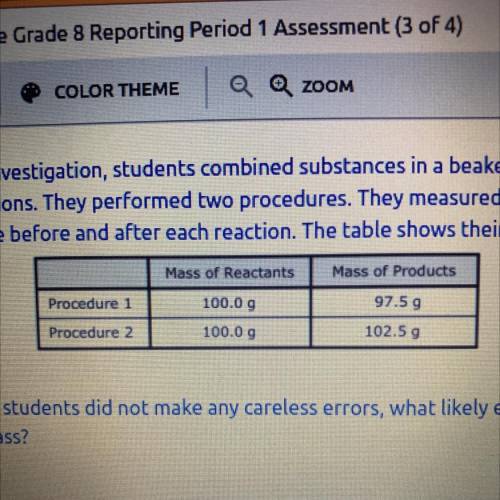
As part of an investigation, students combined substances in a beaker to observe
chemical reactions. They performed two procedures. They measured the mass of
each substance before and after each reaction. The table shows their observations.
Mass of Products
Procedure
97.59
Procedure 2
102.50
Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into
the air
Mass of Reactants
100.0 9
100.00
Procedure 1: One of the reactants was converted to thermal
energy,
Procedure 2: All the products were liquids.
e
Assuming the students did not make any careless errors, what likely explains these
changes in mass?
Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
e
air.
Procedure 1: One of the products was a gas that escaped into the
Procedure 2: A gas from the air reacted with one of the other
reactants and formed a precipitate.
CLEAR ALL

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
As part of an investigation, students combined substances in a beaker to observe
chemical reactions...
Questions

Mathematics, 25.07.2019 09:40

Mathematics, 25.07.2019 09:40



History, 25.07.2019 09:40

Mathematics, 25.07.2019 09:40

Mathematics, 25.07.2019 09:40



Mathematics, 25.07.2019 09:40



Mathematics, 25.07.2019 09:40

Mathematics, 25.07.2019 09:40



Mathematics, 25.07.2019 09:40

Computers and Technology, 25.07.2019 09:40

Computers and Technology, 25.07.2019 09:40




