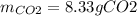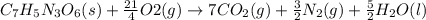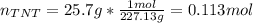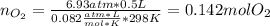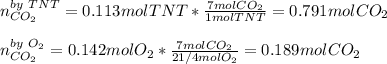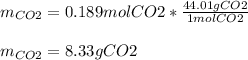Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced chemical equation:
C7H5N3O6(s)+214O2(g)→7CO2(g)+32N2(g )+52H2O(l)
If 25.7 g of TNT is combusted in a 0.500 L container filled with O2 at a pressure of 7.02 bar and a temperature of 298 K, calculate the maximum mass of CO2 that could be produced.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced ch...
Questions


Biology, 14.07.2020 22:01


Physics, 14.07.2020 22:01



English, 14.07.2020 22:01




Mathematics, 14.07.2020 22:01


Mathematics, 14.07.2020 22:01



Mathematics, 14.07.2020 22:01

Computers and Technology, 14.07.2020 22:01



History, 14.07.2020 22:01