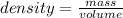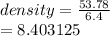Chemistry, 06.11.2020 06:30 chonawilson4
When a solid with a mass of 53.78 g is added to 50.0 ml of water in a graduated cylinder, the volume increases to 56.4 ml. Calculate the density of the solid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
When a solid with a mass of 53.78 g is added to 50.0 ml of water in a graduated cylinder, the volume...
Questions

History, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31


Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31


Social Studies, 28.01.2020 09:31



Social Studies, 28.01.2020 09:31




Business, 28.01.2020 09:31


Chemistry, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31