

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
What is the concentration of silver ions in a saturated solution of Ag3PO4? Ksp (Ag3PO4) = 8.9 x 10...
Questions

Mathematics, 14.10.2019 19:30





Mathematics, 14.10.2019 19:30




History, 14.10.2019 19:30

Social Studies, 14.10.2019 19:30



Geography, 14.10.2019 19:30

Mathematics, 14.10.2019 19:30

History, 14.10.2019 19:30


History, 14.10.2019 19:30

Advanced Placement (AP), 14.10.2019 19:30

English, 14.10.2019 19:30

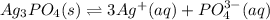
![Ksp=[Ag^+]^3[PO_4^{3-}]](/tpl/images/0874/3681/0c314.png)
 , we can write:
, we can write: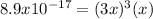
![x=\sqrt[4]{\frac{8.9x10^{-17}}{27} } \\\\x=4.3x10^{-5}M](/tpl/images/0874/3681/6dae2.png)


