
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling point of - 1. °C.
Suppose the pressure on a 3.0 m3 sample of butane gas at 19.0°C is reduced to one-third its initial value.
Is it possible to change the temperature of the butane at the same time such that
the volume of the gas doesn't change?
If you answered yes, calculate the new temperature of the gas. Round your
answer to the nearest °C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling poin...
Questions

Mathematics, 05.05.2020 02:28

Social Studies, 05.05.2020 02:28

Mathematics, 05.05.2020 02:28

History, 05.05.2020 02:28


Biology, 05.05.2020 02:29


Arts, 05.05.2020 02:29


Mathematics, 05.05.2020 02:29



History, 05.05.2020 02:29

History, 05.05.2020 02:29

English, 05.05.2020 02:29


Mathematics, 05.05.2020 02:29

History, 05.05.2020 02:29

Mathematics, 05.05.2020 02:29

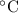 .
.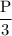
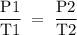
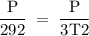
 3T2 = P
3T2 = P 

