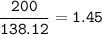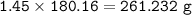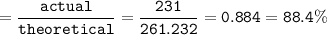PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A student begins a reaction with 200.0 grams of C7H603. Calculate the percent yield of the reaction if 231.0 grams of C9H304 is actually
produced.
C7H. O3 + C4H8O3 C9H204 + C2H4O2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A stu...
Questions

Mathematics, 14.06.2021 19:40




Mathematics, 14.06.2021 19:40

Geography, 14.06.2021 19:40


English, 14.06.2021 19:40




Business, 14.06.2021 19:40




Social Studies, 14.06.2021 19:40

Chemistry, 14.06.2021 19:40