
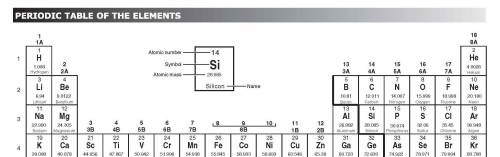
How would an element on the left side of row 2 of the periodic table differ from an element in the middle of the same row?
A. The element on the left would have more atomic mass.
B. The element on the left would have less malleability.
C. The element on the left would have a lower melting point.
D. The element on the left would have no metallic properties.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1


Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
How would an element on the left side of row 2 of the periodic table differ from an element in the m...
Questions



Mathematics, 20.08.2019 08:00

Social Studies, 20.08.2019 08:00

Mathematics, 20.08.2019 08:00

History, 20.08.2019 08:00


Mathematics, 20.08.2019 08:00




Mathematics, 20.08.2019 08:00

Mathematics, 20.08.2019 08:00

Mathematics, 20.08.2019 08:00




History, 20.08.2019 08:00

Geography, 20.08.2019 08:00

French, 20.08.2019 08:00



