
Chemistry, 26.01.2020 01:31 havanaoohnana
To form an oxygen molecule (02), two oxygen atoms share two pairs of electrons. what kind of bond is shown below by the electron dot diagram of 02? choose all that apply.
2 answers
1) covalent bond
2) ionic bond
3) single bond
4) double bond
5) triple bond
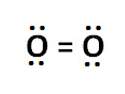

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
To form an oxygen molecule (02), two oxygen atoms share two pairs of electrons. what kind of bond is...
Questions

Computers and Technology, 28.07.2019 04:00

Mathematics, 28.07.2019 04:00

Mathematics, 28.07.2019 04:00

Biology, 28.07.2019 04:00


Chemistry, 28.07.2019 04:00






Mathematics, 28.07.2019 04:00



Chemistry, 28.07.2019 04:00


Mathematics, 28.07.2019 04:00





