Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
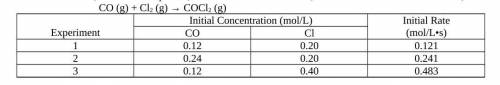
Using the experimental data provided, determine the order of reaction with respect to each reactant,...
Questions

Social Studies, 11.07.2019 09:30



English, 11.07.2019 09:30

Business, 11.07.2019 09:30

English, 11.07.2019 09:30


Mathematics, 11.07.2019 09:30


Computers and Technology, 11.07.2019 09:30

Mathematics, 11.07.2019 09:30

Chemistry, 11.07.2019 09:30



World Languages, 11.07.2019 09:30

English, 11.07.2019 09:30


Mathematics, 11.07.2019 09:30

Health, 11.07.2019 09:30