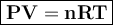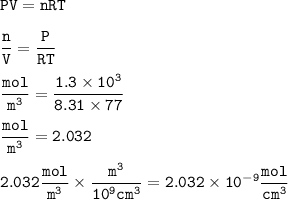Chemistry, 08.11.2020 08:20 jhjhgjvygv
vacuum line is lowered to a pressure of 1.3kpa and 77 k find the number of moles per mm3. Assume that R= 8.31 JK-1 mol-1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
vacuum line is lowered to a pressure of 1.3kpa and 77 k find the number of moles per mm3. Assume tha...
Questions

Computers and Technology, 15.02.2022 14:00


Social Studies, 15.02.2022 14:00







Mathematics, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

History, 15.02.2022 14:00




Chemistry, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00


Social Studies, 15.02.2022 14:00