Example for next four problems:
Compound formula: MgCl2
Element: Mg
# of At...

Chemistry, 08.11.2020 14:00 kenleighbrooke67
Example for next four problems:
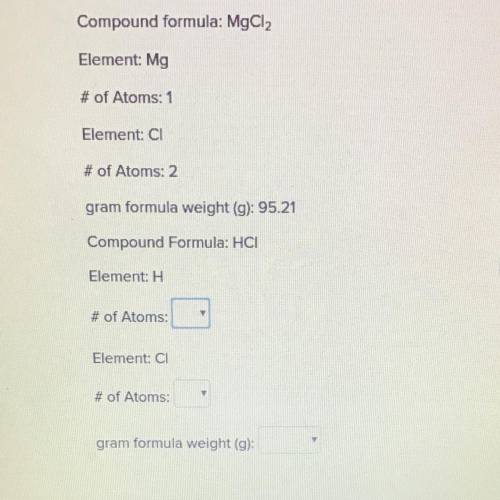
Compound formula: MgCl2
Element: Mg
# of Atoms: 1
Element: Cl
# of Atoms: 2
gram formula weight (g): 95.21
Compound Formula: HCI
Element H
# of Atoms: (Options are 1, 2, and 3)
Element: CI
# of Atoms: (Options are 1, 2, and 3)
gram formula weight (g): (Options are 36, 36.46, and 36.50)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Questions

Mathematics, 14.07.2019 18:30




Chemistry, 14.07.2019 18:30



Mathematics, 14.07.2019 18:30



Mathematics, 14.07.2019 18:30


History, 14.07.2019 18:30




Arts, 14.07.2019 18:30



Physics, 14.07.2019 18:30



