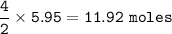Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3



You know the right answer?
The combustion of ethane (C 2 H 6 ) produces carbon dioxide and steam.
2C 2 H 6 (g)+7O 2 (g) 4CO 2...
Questions

Health, 25.08.2019 13:30


Mathematics, 25.08.2019 13:30

Health, 25.08.2019 13:30

History, 25.08.2019 13:30

History, 25.08.2019 13:30


History, 25.08.2019 13:30

Biology, 25.08.2019 13:30





History, 25.08.2019 13:30

Biology, 25.08.2019 13:30

English, 25.08.2019 13:30

Social Studies, 25.08.2019 13:30

History, 25.08.2019 13:30