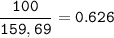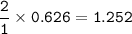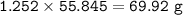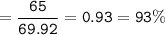Chemistry, 08.11.2020 16:40 athenajames1221
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculate the maximum theoretical mass of iron that can be made from 100g
of iron oxide.
b) In the reaction, only 65 g of iron was made. Calculate the percentage yield.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculat...
Questions


History, 24.03.2020 01:29



Biology, 24.03.2020 01:29





Mathematics, 24.03.2020 01:29

Mathematics, 24.03.2020 01:29

Mathematics, 24.03.2020 01:29



Social Studies, 24.03.2020 01:30

Chemistry, 24.03.2020 01:30

Physics, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30