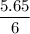Chemistry, 09.11.2020 01:50 Rocket3138
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the following reaction. Both reactant gases are initially at STP B2H6(g) + 6 Cl2(g) —> 3 BCl3(g) + 6HCl(g) Delta H = -1396 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3


Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the fo...
Questions



Computers and Technology, 30.06.2021 18:20







History, 30.06.2021 18:20

Mathematics, 30.06.2021 18:20


Chemistry, 30.06.2021 18:20

Mathematics, 30.06.2021 18:20


Mathematics, 30.06.2021 18:20

Social Studies, 30.06.2021 18:20

Mathematics, 30.06.2021 18:20

Mathematics, 30.06.2021 18:20

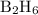 has been 58.637 kJ.
has been 58.637 kJ.