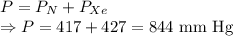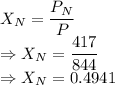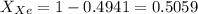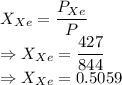Chemistry, 09.11.2020 16:20 allenpaietonp9v8sv
A mixture of nitrogen and xenon gases contains nitrogen at a partial pressure of 417 mm Hg and xenon at a partial pressure of 427 mm Hg. What is the mole fraction of each gas in the mixture

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
A mixture of nitrogen and xenon gases contains nitrogen at a partial pressure of 417 mm Hg and xenon...
Questions


Mathematics, 14.02.2022 06:30


Mathematics, 14.02.2022 06:30

Mathematics, 14.02.2022 06:30

Mathematics, 14.02.2022 06:30


Mathematics, 14.02.2022 06:30


Mathematics, 14.02.2022 06:40






Mathematics, 14.02.2022 06:40




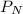 = Partial pressure of nitrogen = 417 mm Hg
= Partial pressure of nitrogen = 417 mm Hg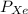 = Partial pressure of xenon = 427 mm Hg
= Partial pressure of xenon = 427 mm Hg