

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Calculate the pH at 25 degrees celsius of a 0.39 M solution of pyridinium chloride (c5h5nhcl) . Note...
Questions

Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01


Mathematics, 14.07.2020 23:01


Biology, 14.07.2020 23:01

English, 14.07.2020 23:01



Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01


Mathematics, 14.07.2020 23:01


Social Studies, 14.07.2020 23:01


Spanish, 14.07.2020 23:01

History, 14.07.2020 23:01


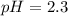
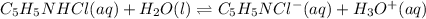
![Ka=\frac{[C_5H_5NCl^-][H_3O^+]}{[C_5H_5NHCl]}](/tpl/images/0880/2037/d1e08.png)
 , we write:
, we write: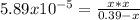
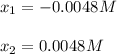
![pH=-log([H_3O^+])=-log(0.0048)\\\\pH=2.3](/tpl/images/0880/2037/04092.png)


