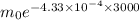Chemistry, 09.11.2020 17:00 coolusername1314
The half life of radium-226 is 1600 years.
a. After how many half-lives would 25% of the radium-226 remain.
b. Write a function m(t) that models the mass remaining after years.
c. How much of the sample will remain after 3000 years?
d. After how long will only 15 mg of the sample remain?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
The half life of radium-226 is 1600 years.
a. After how many half-lives would 25% of the radium-226...
Questions



Mathematics, 31.05.2020 23:59


Mathematics, 31.05.2020 23:59





Mathematics, 31.05.2020 23:59


Mathematics, 31.05.2020 23:59

Business, 31.05.2020 23:59

Mathematics, 31.05.2020 23:59




Mathematics, 31.05.2020 23:59