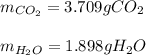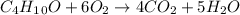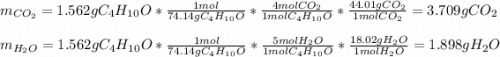Chemistry, 09.11.2020 17:20 dannyo6680
1.562 g sample of the alcohol CH3CHOHCH2CH3 is burned in an excess of oxygen. What masses of H2O and CO2 should be obtained

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1



Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1
You know the right answer?
1.562 g sample of the alcohol CH3CHOHCH2CH3 is burned in an excess of oxygen. What masses of H2O and...
Questions

Mathematics, 01.09.2021 21:20

Biology, 01.09.2021 21:20

Mathematics, 01.09.2021 21:20

Mathematics, 01.09.2021 21:20

Mathematics, 01.09.2021 21:20


Computers and Technology, 01.09.2021 21:20


Mathematics, 01.09.2021 21:20


Chemistry, 01.09.2021 21:20


Mathematics, 01.09.2021 21:20


Mathematics, 01.09.2021 21:20

Mathematics, 01.09.2021 21:20


Mathematics, 01.09.2021 21:20