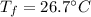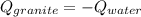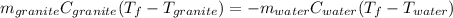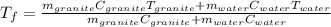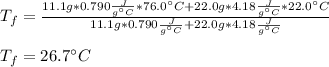Chemistry, 09.11.2020 17:20 alysonmariefont
A 11.1-g sample of granite initially at 76.0°C is immersed into 22.0 g of water initially at 22.0°C. What is the final temperature of both substances when they reach thermal equilibrium? (For water, Cs=4.18J/g⋅∘C and for granite, Cs=0.790J/g⋅∘C.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3


You know the right answer?
A 11.1-g sample of granite initially at 76.0°C is immersed into 22.0 g of water initially at 22.0°C....
Questions




Mathematics, 05.05.2020 16:39

History, 05.05.2020 16:39

Mathematics, 05.05.2020 16:39


Mathematics, 05.05.2020 16:39

Spanish, 05.05.2020 16:39


Mathematics, 05.05.2020 16:39


History, 05.05.2020 16:39




Social Studies, 05.05.2020 16:39