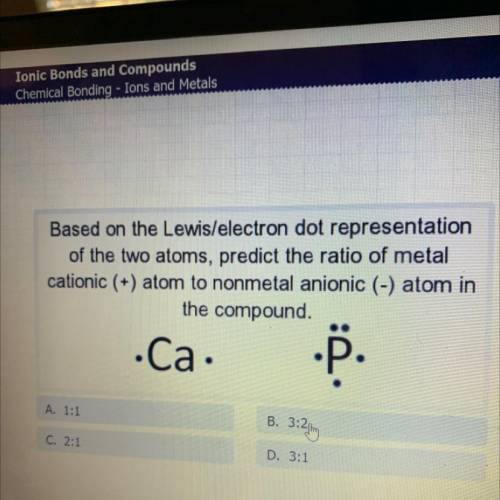
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
c...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3
You know the right answer?
Questions

English, 22.12.2020 15:10


English, 22.12.2020 15:10


Mathematics, 22.12.2020 15:10

Mathematics, 22.12.2020 15:10

Mathematics, 22.12.2020 15:10

Social Studies, 22.12.2020 15:10

Physics, 22.12.2020 15:10

English, 22.12.2020 15:10

Mathematics, 22.12.2020 15:10


Biology, 22.12.2020 15:20

Spanish, 22.12.2020 15:20

Biology, 22.12.2020 15:20



Mathematics, 22.12.2020 15:20