
Chemistry, 09.11.2020 19:20 mariam00000w
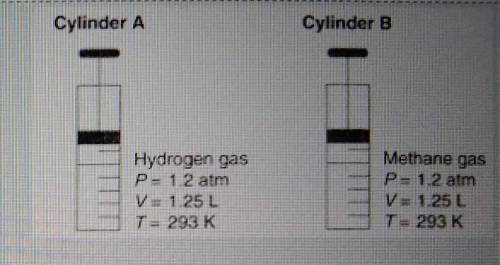
Diagram shows that both gases occupy the same volume under the same conditions of temperature and pressure. Show a numerical set up for how you will calculate the new volume of the gas, if the pressure remains constant ( at 1.2atm), but the temperature is raised from 293k to 398K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2


Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Diagram shows that both gases occupy the same volume under the same conditions of temperature and pr...
Questions


History, 12.08.2020 07:01





Mathematics, 12.08.2020 07:01



Mathematics, 12.08.2020 07:01

English, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01


English, 12.08.2020 07:01



Mathematics, 12.08.2020 07:01



