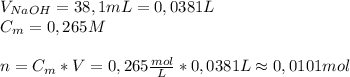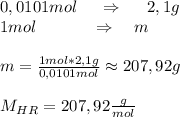Chemistry, 29.08.2019 10:10 JFrocks2480
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass of the ac? i already calculated that there are .0101 moles of naoh so that i have .0101 mol of the unknown acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass...
Questions




Computers and Technology, 23.08.2019 02:00


Chemistry, 23.08.2019 02:00



Mathematics, 23.08.2019 02:00

Chemistry, 23.08.2019 02:00

Mathematics, 23.08.2019 02:00

English, 23.08.2019 02:00



English, 23.08.2019 02:00


History, 23.08.2019 02:00

English, 23.08.2019 02:00