
Chemistry, 10.11.2020 01:00 jeremiah1212
Compare the relative strength of the two forces B and C. Explain how you determined this comparison by identifying the forces.
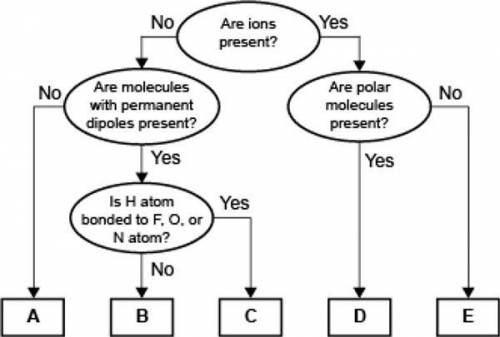

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Compare the relative strength of the two forces B and C. Explain how you determined this comparison...
Questions





Mathematics, 30.10.2020 17:40






Social Studies, 30.10.2020 17:40


Computers and Technology, 30.10.2020 17:40



World Languages, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40


Mathematics, 30.10.2020 17:40



