
Chemistry, 10.11.2020 09:20 Josephjenkins620
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calculate the mass of 3.33 moles of air.
First, complete the unit conversion using dimensional analysis:
A • B/C
A: 3.33 mil air
B: 28.87 g air
C: 1 mol air

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calc...
Questions



Biology, 25.11.2019 03:31

Chemistry, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

Social Studies, 25.11.2019 03:31

History, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31




English, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31




Mathematics, 25.11.2019 03:31


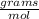 * 3.33 moles
* 3.33 moles

