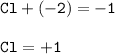9701 s16_qp_12.pdf - Adobe Reader
Edit View Window Help
a Open
Tool
Fill B. Sign<...

Chemistry, 10.11.2020 14:00 gmoney1973
9701 s16_qp_12.pdf - Adobe Reader
Edit View Window Help
a Open
Tool
Fill B. Sign
Comment
143%
9
In the treatment of domestic water supplies, chlorine is added to the water to form HCIO.
Cl2(aq) + H2O(1) ►
H(aq) + Cl(aq) + HCIO(aq)
The HCIO reacts further to give CIO ions.
HCIO(aq) + H2O(l) 7H30*(aq) + CIO (aq)
Both HCIO and C10 kill bacteria by oxidation.
What is the change in oxidation number of chlorine when forming the Cio-ion from aqueous
chlorine?
B 0
D
A-1
C +1
+2
9701/12/M/J/16
© UCLES 2016

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
Questions

Biology, 29.06.2019 13:30




History, 29.06.2019 13:30


Mathematics, 29.06.2019 13:30





Mathematics, 29.06.2019 13:30


Spanish, 29.06.2019 13:30

Mathematics, 29.06.2019 13:30


Mathematics, 29.06.2019 13:30


Mathematics, 29.06.2019 13:30

Mathematics, 29.06.2019 13:30