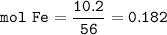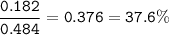Chemistry, 11.11.2020 06:10 connienash95
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a) Actual yield of Fe mole
(b) % mole
(c) theoretical yield of iron mmoles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a)...
(a)...
Questions




Mathematics, 09.12.2021 01:00



Mathematics, 09.12.2021 01:00





Mathematics, 09.12.2021 01:00



Social Studies, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00

Business, 09.12.2021 01:00

English, 09.12.2021 01:00