
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached below)
response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
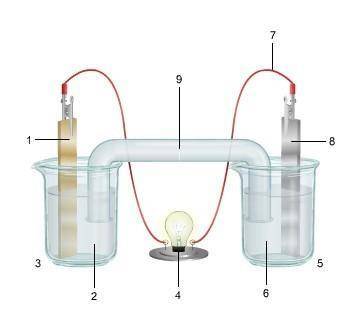

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 22:00
Calculate the partial pressure, in atmospheres, of o2 in the dry air outside an airliner cruising at an altitude of about 20000 ft (6096 m), where the atmoshperic pressure is 351 mm hg. how much must the outside air be compressed to produce a cabin pressure in which the partial pressure of o2 is 0.200 atm?
Answers: 2
You know the right answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached...
Questions

Mathematics, 21.08.2020 20:01



English, 21.08.2020 20:01

Mathematics, 21.08.2020 20:01






Health, 21.08.2020 20:01


Mathematics, 21.08.2020 20:01

Mathematics, 21.08.2020 20:01



Mathematics, 21.08.2020 20:01

Mathematics, 21.08.2020 20:01




