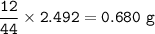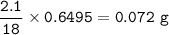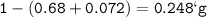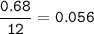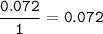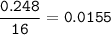Chemistry, 11.11.2020 16:00 HalpMehPlz
A 100g of sample of a compound is combusted in excess oxygen and the products are 2.492g of CO2 and 0.6495 of H2O. Determine the empirical formula of the compound?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
A 100g of sample of a compound is combusted in excess oxygen and the products are 2.492g of CO2 and...
Questions

Mathematics, 27.05.2021 19:40


Mathematics, 27.05.2021 19:40


Advanced Placement (AP), 27.05.2021 19:40


Mathematics, 27.05.2021 19:40

History, 27.05.2021 19:40



Mathematics, 27.05.2021 19:40


Mathematics, 27.05.2021 19:40


German, 27.05.2021 19:40



Mathematics, 27.05.2021 19:40

Mathematics, 27.05.2021 19:40

Health, 27.05.2021 19:40