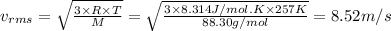Chemistry, 11.11.2020 17:30 joycewingate919
Calculate the root mean square (rms) average speed of the atoms in a sample of krypton gas at 0.14 atm and -16 0C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Calculate the root mean square (rms) average speed of the atoms in a sample of krypton gas at 0.14 a...
Questions







Health, 21.06.2019 14:10



Mathematics, 21.06.2019 14:10

Mathematics, 21.06.2019 14:10

Geography, 21.06.2019 14:10




History, 21.06.2019 14:10


Mathematics, 21.06.2019 14:10

History, 21.06.2019 14:10