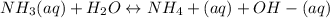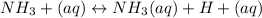A) Earlier you were told that of all of the 14 solutes you will be studying, the only one that is not appreciably ionized in water is aqueous ammonia. What does this statement imply about the equilibrium point of the reaction involving aqueous ammonia/ammonium hydroxide?B) Use your results and conclusions from part (a) to explain why a complicated name like Aqueous ammonia/ammonium hydroxide is used for this solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1


Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
A) Earlier you were told that of all of the 14 solutes you will be studying, the only one that is no...
Questions

Mathematics, 21.03.2021 23:40


Mathematics, 21.03.2021 23:40


Mathematics, 21.03.2021 23:40

History, 21.03.2021 23:40

English, 21.03.2021 23:40



Mathematics, 21.03.2021 23:40


Mathematics, 21.03.2021 23:40

Computers and Technology, 21.03.2021 23:40



Computers and Technology, 21.03.2021 23:50


History, 21.03.2021 23:50

Mathematics, 21.03.2021 23:50

World Languages, 21.03.2021 23:50