Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized in the gas phase by the reaction of gas phase carbon monoxide with gas phase hydrogen. A 10.0 L reaction flask contains carbon monoxide gas at 0.461 bar and 22.0 °C. 345 mL of hydrogen gas at 7.12 bar and 271 K is introduced. Assume the reaction goes to completion (100% yield). What are the partial pressures of each gas at the end of the reaction, once the temperature has returned to22.0 °C?. units in bar please

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3


You know the right answer?
Please answer I have a finals and I need help with a question similar!!! Methanol can be synthesized...
Questions







History, 10.03.2020 00:57

Mathematics, 10.03.2020 00:57

Mathematics, 10.03.2020 00:57



Mathematics, 10.03.2020 00:57






Mathematics, 10.03.2020 00:57

Biology, 10.03.2020 00:57

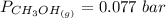
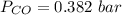
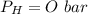
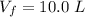
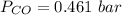
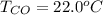
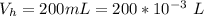
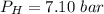
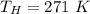
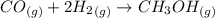
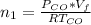
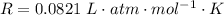
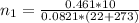
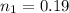
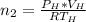
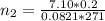
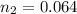
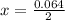
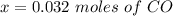

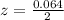
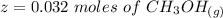
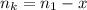
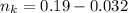
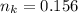
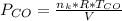
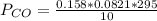
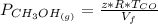
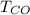 is used because it is given the question that the temperature returned to 22.0 degrees C
is used because it is given the question that the temperature returned to 22.0 degrees C