
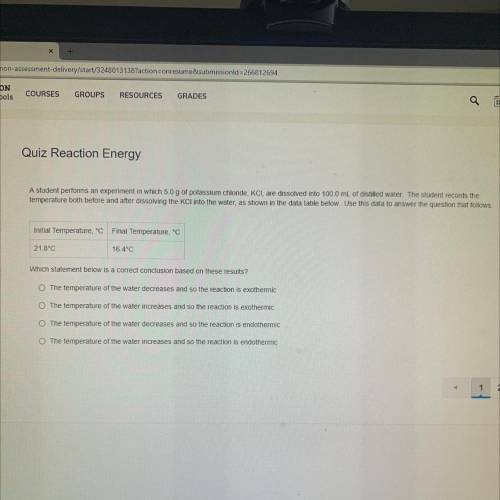
A student performs an experiment in which 5.0 g of potassium chloride, KCI, are dissolved into 100.0 mL of distilled water. The student records the
temperature both before and after dissolving the KCl into the water, as shown in the data table below. Use this data to answer the question that follows.
Initial Temperature, °C Final Temperature, °C
21.8°C
16.4°C
Which statement below is a correct conclusion based on these results ?
The temperature of the water decreases and so the reaction is exothermic
The temperature of the water increases and so the reaction is exothermic
The temperature of the water decreases and so the reaction is endothermic
The temperature of the water increases and so the reaction is endothermic

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
A student performs an experiment in which 5.0 g of potassium chloride, KCI, are dissolved into 100.0...
Questions

Arts, 02.04.2021 22:30


Spanish, 02.04.2021 22:30


Mathematics, 02.04.2021 22:30

English, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30

History, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30

History, 02.04.2021 22:30

History, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30





Mathematics, 02.04.2021 22:30


Mathematics, 02.04.2021 22:30



