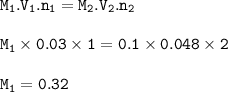Chemistry, 12.11.2020 04:40 Silkyruthie
What is the molarity of a hydrochloric acid solution, when 30.0 mL is neutralized by 48.0 mL of 0.100 mol/L calcium hydroxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
What is the molarity of a hydrochloric acid solution, when 30.0 mL is neutralized by 48.0 mL of 0.10...
Questions



Mathematics, 09.04.2020 18:13


Mathematics, 09.04.2020 18:13

Mathematics, 09.04.2020 18:13





Mathematics, 09.04.2020 18:13




Mathematics, 09.04.2020 18:13

Advanced Placement (AP), 09.04.2020 18:13