Chemistry, 12.11.2020 05:50 F00Dislife
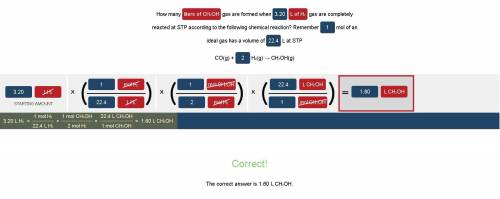
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP according to the following chemical reaction?
Remember 1 mol of an ideal gas has a volume of 22.4 L at STP
CO(g) + 2 H₂(g) → CH₃OH(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP accordin...
Questions


English, 02.07.2019 18:10


Mathematics, 02.07.2019 18:10



Mathematics, 02.07.2019 18:10


English, 02.07.2019 18:10

History, 02.07.2019 18:10

History, 02.07.2019 18:10


Mathematics, 02.07.2019 18:10

Mathematics, 02.07.2019 18:10


Geography, 02.07.2019 18:10