
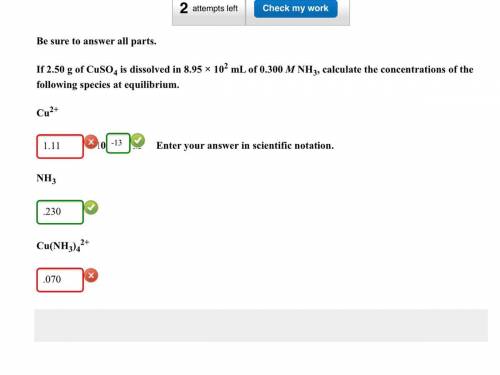
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the following species at equilibrium: Cu^2+, NH3, Cu(NH3)4^2.
I tried to solve and came up with the following-
Cu^2+ = 1.1149x10^-13 (Which was all wrong except for the 10^-13)
NH3 = .230 (Which was correct)
Cu(NH3)4^2+ = 0.069720 (Which was wrong)
Can someone please show me where I am going wrong.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

You know the right answer?
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the...
Questions

Mathematics, 03.04.2020 01:23

Biology, 03.04.2020 01:23


English, 03.04.2020 01:24

Mathematics, 03.04.2020 01:24


Mathematics, 03.04.2020 01:24

Mathematics, 03.04.2020 01:24

Mathematics, 03.04.2020 01:24

Mathematics, 03.04.2020 01:24

English, 03.04.2020 01:24



English, 03.04.2020 01:24

Chemistry, 03.04.2020 01:24




History, 03.04.2020 01:24

Mathematics, 03.04.2020 01:24



