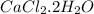Chemistry, 29.08.2019 07:00 mimireds5419
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within the desiccators and forms a hydrated salt. a 16.43g mass of anhydrous cacl2 weighed 21.75g after being left in desiccators for several months. what is the formula of the hydrated cacl2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within th...
Questions


Spanish, 14.12.2021 17:40



Mathematics, 14.12.2021 17:40



Geography, 14.12.2021 17:40



Computers and Technology, 14.12.2021 17:40

Computers and Technology, 14.12.2021 17:40

History, 14.12.2021 17:40