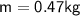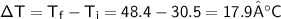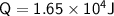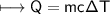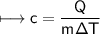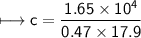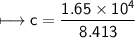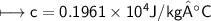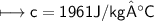Chemistry, 13.11.2020 09:10 naeaamm2528
A chemist carefully measures the amount of heat needed to raise the temperature of a 0.47 kg sample of C6H7N from 30.5 degrees C to 48.4 degrees C. The experiment shows that 1.65 x 10^4 J of heat are needed. What can the chemist report for the molar heat capacity of C6H7N? Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
A chemist carefully measures the amount of heat needed to raise the temperature of a 0.47 kg sample...
Questions



History, 27.08.2019 22:00



Mathematics, 27.08.2019 22:00


Mathematics, 27.08.2019 22:00




Biology, 27.08.2019 22:00


Social Studies, 27.08.2019 22:00



English, 27.08.2019 22:00

Mathematics, 27.08.2019 22:00

Physics, 27.08.2019 22:00