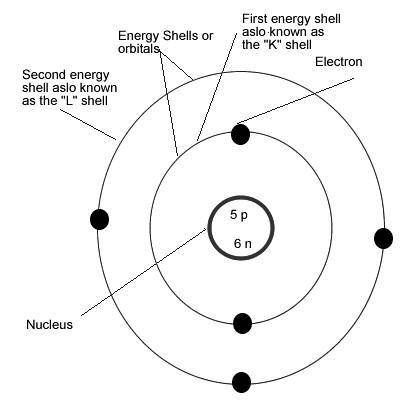
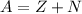Which of the following statements about elements is not true?
A. Elements contain equal numbers of protons and electrons.
B. Elements contain equal numbers of neutrons and electrons.
C. Elements contain protons and neutrons in their nucleus.
D. Elements contain electrons that orbit around the nucleus.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Which of the following statements about elements is not true?
A. Elements contain equal numbers of...
Questions


English, 23.07.2021 14:00


Chemistry, 23.07.2021 14:00


Chemistry, 23.07.2021 14:00



Mathematics, 23.07.2021 14:00


Mathematics, 23.07.2021 14:00


SAT, 23.07.2021 14:00

Social Studies, 23.07.2021 14:00


Chemistry, 23.07.2021 14:00


Spanish, 23.07.2021 14:00

Chemistry, 23.07.2021 14:00