
Chemistry, 13.11.2020 23:20 vanessa791
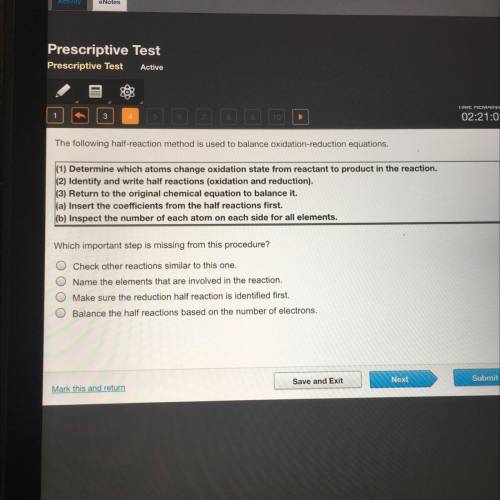
The following half-reaction method is used to balance oxidation-reduction equations.
(1) Determine which atoms change oxidation state from reactant to product in the reaction.
(2) Identify and write half reactions (oxidation and reduction).
(3) Return to the original chemical equation to balance it.
(a) Insert the coefficients from the half reactions first.
(b) Inspect the number of each atom on each side for all elements.
Which important step is missing from this procedure?
Check other reactions similar to this one.
Name the elements that are involved in the reaction.
Make sure the reduction half reaction is identified first.
Balance the half reactions based on the number of electrons.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1
You know the right answer?
The following half-reaction method is used to balance oxidation-reduction equations.
(1) Determine...
Questions

Chemistry, 19.03.2021 01:00


Mathematics, 19.03.2021 01:00

Mathematics, 19.03.2021 01:00

English, 19.03.2021 01:00




Biology, 19.03.2021 01:00


English, 19.03.2021 01:00


Social Studies, 19.03.2021 01:00

Mathematics, 19.03.2021 01:00



Mathematics, 19.03.2021 01:00

Mathematics, 19.03.2021 01:00

Mathematics, 19.03.2021 01:00



